Heat-Resistant and Signal-Stable: Why Polyimide (PI) is the Invisible Hero of Semiconductors
On a scorching summer day, a phone left in a hot car continues to function normally, with 5G signal holding steady. Or consider a foldable phone screen that bends freely without damage.
Behind these miracles of withstanding high heat without “gasping” and maintaining signal integrity stands a silent material hero—Polyimide (PI). As a high-performance polymer, PI is widely used in various electronic and electrical devices, from motor insulation, high-temperature capacitors, and transformers to the flexible screens we use daily.
It always works behind the scenes to ensure stable device operation, yet rarely receives attention. Thus, PI rightfully earns the title of the “invisible hero” in the semiconductor field.
I. Material Properties of PI (Polyimide)
Exceptional Thermal Stability
PI possesses extremely high thermal stability, not only enduring high temperatures but also maintaining its properties at low temperatures. Typical aromatic polyimides have glass transition temperatures (Tg) often exceeding 200°C, and decomposition temperatures can reach around 500°C, allowing them to remain “unfazed” in harsh thermal environments.
For instance, some PI aerogels have been shown to remain stable across a wide temperature range from -60°C to 470°C. This outstanding heat resistance makes PI the ideal choice for aerospace and high-temperature electronic components.
Excellent Electrical Insulation Properties
The imide ring in the polyimide molecular structure contains polar carbonyl groups. However, these carbonyl groups are distributed in a conjugated and symmetric manner, making it difficult for free electrons to migrate, thereby endowing PI with excellent electrical insulation properties.
Its dielectric constant (k) is approximately 3.4 for standard grades, and its dielectric breakdown strength is remarkably high, exceeding 300 kV/mm. This means PI films are resistant to breakdown even under high electric fields, effectively ensuring signals remain “undistorted and uncrossed.” It’s no wonder PI is one of the most important interlayer insulating materials in microelectronic devices.
Good Toughness and Fatigue Resistance
Despite being part of the plastic family, PI’s mechanical strength and rigidity are comparable to some metals. The rigid backbone structure of aromatic PI gives it a high Young’s modulus (typically in the 2–5 GPa range, often >2.5 GPa), along with good toughness and fatigue resistance.
Even in extreme environments such as high temperatures and high radiation, PI materials maintain their mechanical strength and dimensional stability without softening or becoming brittle. For example, PI aerogel membranes reinforced with PI nanofibers can achieve a tensile strength of 2.95 MPa with a density of only 0.168 g/cm³ (compared to 0.27 MPa for pure PI aerogels), demonstrating both strength and light weight.
II. Embodiment of Polymer Physics Wisdom in Polyimide
The Rigid Molecular Backbone is the Root Cause of PI’s High Thermal Stability and Strength
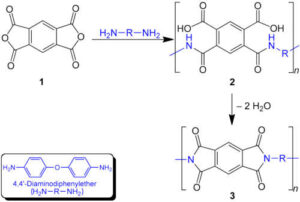
PI’s main chain contains numerous aromatic rings and imide hexatomic rings. These structures are like inserting “steel bars” into the molecular chains, significantly increasing chain rigidity.
Rigid chain segments are difficult to bend and rotate, which inhibits molecular motion when heated. Consequently, the glass transition temperature (Tg) is much higher than that of typical polymers.
Most aromatic PIs have a Tg between 250–400°C, far exceeding common engineering plastics like polycarbonate (~150°C). A high Tg means that within the operating range from room temperature to high temperatures, PI chains remain in a glassy state, with molecules almost “immobile,” so the material retains its hardness and strength without softening due to temperature increase.
Additionally, the chemical bonds in aromatic imides have high bond energy, and the chains have few unsaturated bonds, making them less prone to thermal decomposition at high temperatures. This explains PI’s decomposition temperatures often exceeding 500°C. In short: the rigid and stable molecular skeleton allows PI to stand “unwavering” before high heat.
Symmetry and Conjugation in Molecular Structure Create PI’s Electrical Insulation Characteristics
Although PI molecules contain polar groups like C=O, these carbonyl groups are paired and embedded within conjugated imide rings. Their positive and negative charge centers cancel each other out, akin to a tug-of-war where both sides are equally matched, resulting in the rope staying centered.
This symmetric conjugated structure greatly restricts the movement of free charges and orientation polarization within the molecule. Therefore, when an external electric field is applied to PI, significant polarization response is difficult to generate within the molecule, keeping the dielectric constant low and the dielectric loss minimal.
This is the microscopic reason for PI’s excellent insulating properties: the molecules are “electrostatically well-behaved,” leading to macroscopic non-conductivity. Precisely for this reason, PI is commonly used in applications requiring insulation, such as capacitor dielectric films and chip dielectric layers, ensuring electrical signals follow their intended paths without interference.
Tight Molecular Packing and Strong Interactions Endow PI with High Strength but Also Present Processing Challenges
Aromatic PI chains often exhibit strong van der Waals forces and π-π interactions between them. The chains tend to stack orderly, further enhancing material rigidity and strength. However, this also makes PI a “stubborn” molecule among polymers: it is difficult to dissolve and melt.
The overly strong intermolecular forces prevent it from flowing easily at high temperatures, which is why traditional PI is difficult to process via thermoplastic methods. On the other hand, modifications to the molecular structure lead to trade-offs in properties.
For example, introducing bulky side groups or non-coplanar rigid units (such as fluorine-containing groups, sterically hindered groups, or alicyclic structures) can reduce PI’s polarizability and increase free volume, thereby lowering the dielectric constant and improving solubility and transparency. But these modifications also disrupt the conjugated rigidity of the main chain, increasing chain flexibility and loosening molecular packing, which can reduce the material’s mechanical strength and thermal stability.
“You can’t have your cake and eat it too” applies to PI as well: structural modifications aimed at lowering the dielectric constant and increasing flexibility often come at the cost of some mechanical strength and heat resistance. The principle of balancing rigidity and flexibility in polymer physics is vividly embodied in PI material modifications.
III. How is Polyimide Prepared?
Diamine-Dianhydride Polycondensation (Linear Polycondensation)
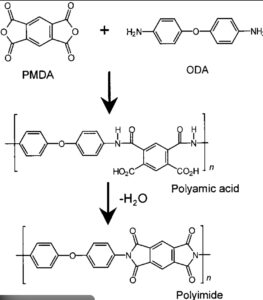
The vast majority of PIs are synthesized via the polycondensation of dianhydrides (e.g., pyromellitic dianhydride, PMDA) with diamines (e.g., 4,4′-oxydianiline, ODA) in a solvent.
The first step typically involves a ring-opening addition reaction at room temperature, forming a polyamic acid (PAA) precursor solution. This process is known as the first step of the DuPont “two-step method.”
The resulting PAA has high molecular weight and contains uncyclized structures of anhydride and amine, forming the basis for subsequent film formation and cyclization.
Imidization (Cyclization Reaction)
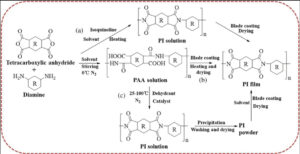
The PAA precursor must be converted into the final polyimide through imidization. There are two main methods for imidization: Thermal imidization involves heating PAA coatings or moldings gradually to temperatures above 200–300°C. This thermal treatment causes intramolecular dehydration and cyclization, forming the rigid imide rings.
This method is simple and yields a high degree of imidization but requires high temperatures and long durations. Chemical imidization involves adding a dehydration cyclization reagent (e.g., an acetic anhydride-pyridine system) to the PAA solution under milder conditions (room temperature to moderate heat).
The chemical reagent reacts with PAA, instantly cyclizing it into PI. The advantage of the chemical method is lower temperature and less shrinkage, but it requires subsequent removal of byproducts and solvents.
Whether thermal or chemical, the goal is to obtain high-molecular-weight linear polyimide. Once this step is complete, the PI material possesses its final molecular structure.
IV. Application Prospects
Semiconductor Dielectric Films
In integrated circuit (IC) manufacturing, PI films have long been used as interlayer dielectric (ILD) materials, insulating adjacent metal interconnects. PI’s thermal stability and mechanical toughness prevent chips from cracking or deforming during processing and operation, while its insulating properties ensure signal integrity without crosstalk.
However, as chip dimensions shrink to the nanoscale, the dielectric constant (k ~3.4) of traditional aromatic PI is now considered relatively high. An excessively high k value leads to signal delay and increased power consumption.
Therefore, significant R&D effort is focused on developing new PI formulations with lower dielectric constants (target k < 3.0, even < 2.0). This involves introducing fluorine atoms, aliphatic structures, or creating micropores to reduce polarizability and dielectric density.
Low-k polyimide films are seen as crucial materials for next-generation very-large-scale integration (VLSI). They can effectively reduce RC delay and power consumption, meeting the performance enhancement demands of chips under Moore’s Law.
Flexible Electronics and Displays
Another major application for polyimide films is in flexible electronics. Thanks to its combination of flexibility and durability in film form, PI is widely used in emerging fields like flexible displays and wearable electronics.
For instance, AMOLED foldable phones use PI films as display substrates, which remain intact despite repeated bending. PI films provide sufficient mechanical support and electrical insulation even at extremely thin thicknesses, while also withstanding high-temperature processes in screen manufacturing (such as the evaporation process for organic light-emitting materials).
More remarkably, PI’s coefficient of thermal expansion is close to that of inorganic materials like silicon, ensuring dimensional stability with temperature changes—a critical factor for multi-layer flexible electronic structures. Currently, transparent or color-tunable polyimide films are also under development for next-generation flexible displays and electronic paper.
It is foreseeable that PI will continue to serve as the “armor” and “backbone” for flexible electronic devices, making future electronics both thin/light and robust/durable.
As the “invisible hero” of the semiconductor field, polyimide, with its high thermal stability, excellent insulation, high strength, and designable structure, plays an irreplaceable role in the electronics industry and even in aerospace. It enables electronic devices to operate without “gasping for breath” under high temperatures and ensures reliable signal transmission in complex environments.
Facing the demands of future technological development, PI is also evolving: researchers are endowing it with intelligent features like self-healing and recyclability through methods such as introducing dynamic covalent bonds, aiming to improve material sustainability without sacrificing traditional excellent performance. Simultaneously, the pursuit of performance limits, such as lowering the dielectric constant and improving thermal conductivity, drives the continuous iteration and optimization of PI.
It is foreseeable that in increasingly extreme scenarios like high-temperature electronics, 5G communications, flexible devices, and deep-space exploration, polyimide will continue to play a crucial role and even realize greater potential. This invisible hero, silently guarding the world of technology, is destined to shine even brighter in the future!
